First disclosure of CATCHFIRE in neurogenesis studies, namely tethering mitochondria and microtubules during the mitosis of neural stemcells. A team led by Evelyne Fischer and Xavier Morin of ENS-PSL (Paris, France) have just disclosed the first application of CATCHFIRE in bioRxiv 2024! Noticeably, this works comes out only one year after the princeps paper by Prof. Arnaud Gautier in Nat. Methods 2023.
“Asymmetric divisions of vertebrate neural progenitors are critical for generating neurons while preserving the stem cell pool, ensuring proper central nervous system development”. Beyond the dogma, a whole world of investigations to decipher the “assymetric”! One certainty, mitochondria have a key role there.
In this study, the authors demonstrate that unequal distribution of mitochondria during asymmetric mitosis plays a decisive role in triggering the differentiation process. They show that daughter cells inheriting fewer mitochondria consistently differentiate into neurons, whereas their sibling receiving more mitochondria retains the progenitor status. Furthermore, implementing CATCHFIRE of The Twinkle Factory, they were able to force the displacement of mitochondria during mitosis towards unequal inheritance, hence driving premature neuronal differentiation. Their findings uncover as a result a key mechanism in neural development… offering a first success to CATCHFIRE.
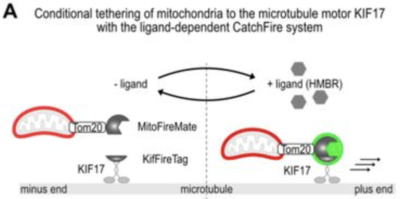
The team have implemented CATCHFIRE to force the tethering of mitochondria to the microtubule motor KIF17. The whole is a ternary system comprising of Mito-FIREmate, Kif-FIREtag and a molecular glue, i.e., HMBR. Not only does the ligand force the assembly of the ternary system, it also allows a fine control of the system, being concentration-dependant and moreover reversible. Not only does the ligand force the assembly of the ternary system, it also allows to report the tethering, getting fluorescent upon assembly (and dark upon disassembly). As a result, the authors found that, within 15 minutes of ligand exposure in mitotic cells, mitochondria located more basally than in controls and appeared more compacted from metaphase until cytokinesis. And, most importantly, unequal mitochondrial segregation was observed…
Our live imaging and fate tracking experiments present the first direct in vivo evidence that mitochondria act as asymmetric cell fate determinants in neural progenitors. Deciphering whether and how mitochondrial segregation is coordinated with […] other [mitotic] determinants […] in spinal progenitors, and exploring the interplay between mitochondria and their downstream pathways, represent challenges for future research.
The Authors
The Twinkle Factory distributes HMBR under the tradename match540, together with match550, match600, and the quencher matchDark. Addgene is your repository of choice for FIREmate and FIREtag plasmids.
Read more about CATCHFIRE and mitochondria
The preprint under review: Unequal mitochondrial segregation promotes asymmetric fates during neurogenesis. , , ,
The princeps paper on CATCHFIRE: A fluorogenic chemically induced dimerization technology for controlling, imaging and sensing protein proximity. Bottone, S., Joliot, O., Cakil, Z.V., El Hajji, L., Rakotoarison, L.-M., Boncompain, G., Perez, F., & Gautier, A. Nat. Methods 20, 1553–1562 (2023). doi.org/10.1038/s41592-023-01988-8
Recent Comments