Monitoring protein tyrosine phosphatases (PTP) activity with FAST and splitFAST. A team led by Amy Barrios of University of Utah have taken the first step toward the development of genetically encodable sensors for PTP activity using FAST. Just published in ChemBioChem 2024.
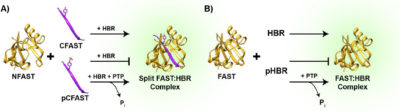
Despite the biological importance and therapeutic potential of PTPs, measuring dynamic intracellular phosphatase activity has so far been challenging. Fluorogenic substrates such as the widely used difluoromethylumbilleferyl phosphate (DiFMUP) have been invaluable for measuring PTP activity in vitro. Now, they are scarcely used for monitoring in vivo intracellular PTP activity. With the goal of expanding the existing toolkit of cell-compatible, fluorogenic assays for PTP activity, the authors were curious about FAST and splitFAST could be compatible fluorogenic PTP substrates.
In this work, the authors effectively demonstrate that FAST can be used as a sensor for PTP-mediated dephosphorylation of phosphorylated dye molecules. For example, they find that while phosphorylated 4-hydroxybenzylidene rhodanine (pHBR) is not able to bind to the FAST protein and induce fluorescence, it provides a sensitive assay for PTP activity, readily detecting 100 pM concentrations of PTP1B in the presence of FAST with a kcat value of 19±1 s−1 and a KM value of 93±3 μM. In addition, while phosphorylation of the C-terminal peptide of split GFP does not result in appreciable change in fluorescence of the reconstituted protein, phosphorylation of the C-terminal peptide of the split FAST protein abrogates fluorescence. Upon PTP-mediated dephosphorylation of the C-terminal peptide, the ability of the N- and C-terminal components to form a fluorescent complex with the small molecule dye is restored, leading to fluorescence.
Those two strategies now need to be tested in vivo! The next steps?
Coming after a variety of embodiments over the latest months, these findings further illustrate the huge versatility of FAST, splitFAST, CATCHFIRE, and their potential in a number of applications. The Twinkle Factory is happy to help!
Read more about tyrosine phosphatase and FAST
Barrios, A., Hansen, D.T., Tu, J., Bouck, A.W. and Mathis, C.L., Multipartite Fluorogenic Sensors for Monitoring Tyrosine Phosphatase Activity. ChemBioChem, p.e202400607.
Recent Comments